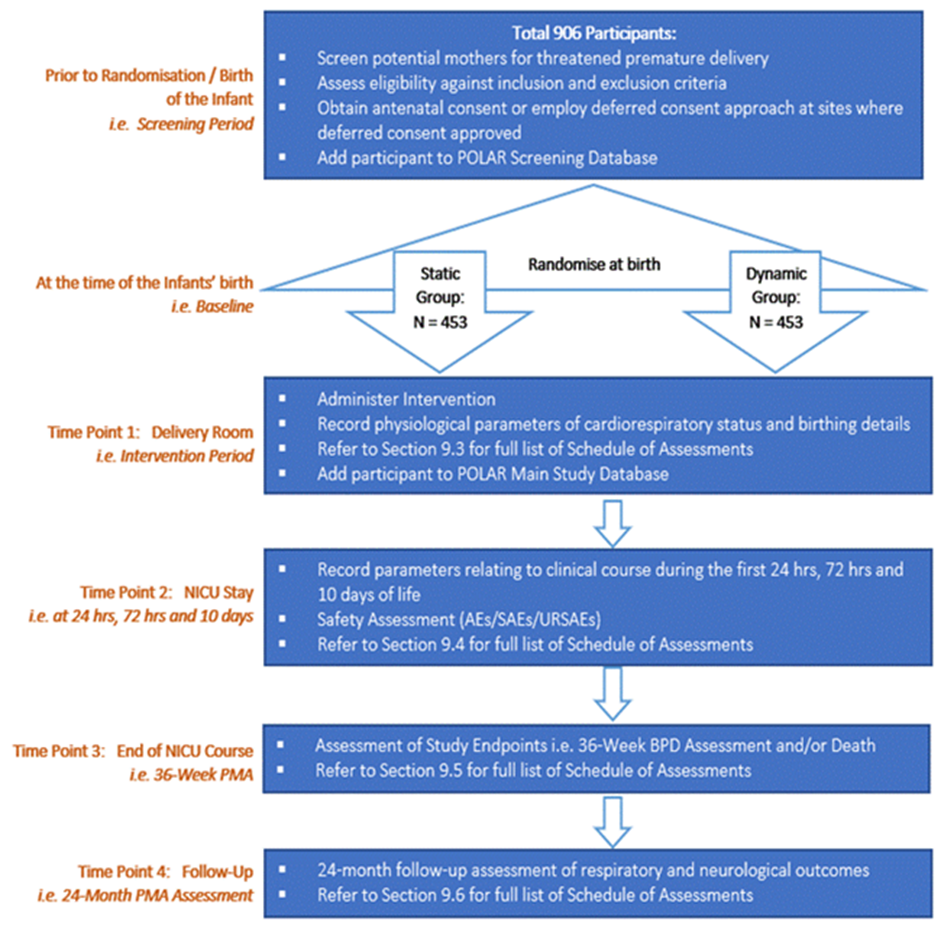
Trial Synopsis & Study Schema
| Trial Title | Positive End-Expiratory Pressure (PEEP) Levels during Resuscitation of Preterm Infants at Birth (The POLAR Trial). |
|---|---|
| Objectives | This trial aims to establish whether the use of a high, dynamic PEEP strategy to support the lung during stabilisation at birth, compared with a static, standard PEEP strategy, increases the rate of survival without bronchopulmonary dysplasia (BPD) in extremely preterm infants born <29 weeks post-menstrual age (PMA). |
| Trial Design | A phase III/IV, international multi-centre prospective randomised control trial to be conducted in the delivery rooms (DRs) of tertiary NICUs experienced in respiratory trials across Australian, European, British, and North American neonatal networks. |
| Trial Outcomes |
The primary outcome is the composite outcome of either death or BPD at 36 weeks Post-Menstrual Age (PMA), as assessed by standard oxygen reduction test. The secondary outcomes include important short-term respiratory morbidity and potential harm outcomes in the DR and NICU. Principal secondary outcomes include:
|
| Trial Population | Preterm infants born between 23 weeks and 0 days and 28 weeks and 6 days PMA (post menstrual age) by best obstetric estimate who are born in participating study centres and require respiratory intervention from birth. |
| Trial Intervention |
Dynamic PEEP Group: Respiratory support with a dynamic PEEP algorithm (8-12 cmH2O) individualised to clinical need. Static PEEP Group: Respiratory support with a static PEEP (5-6 cmH2O) consistent with current Neonatal Resuscitation Program (NRP) guidelines. |
| Number of Participants | 906 infants (453 infants per group) |
| Recruitment Period | Approximately 5 years after first infant randomised |
| Follow Up Period | 24 months |
| Trial Registration |
Clinicaltrials.gov Registry No: NCT04372953 ANZCTR Registry No: ACTRN12618001686291 |
Study Schema